Field Corrective Action (FCA)
What is Field Safety Corrective Action?
Field safety corrective action (FSCA) is a post market requirement that need to be followed by an establishment under Section 41 of Act 737. The establishment is required to take proactive steps to inform the authority as well as their customers/end-users on the corrective actions made to ensure compliance of these requirements.
Manufacturers or their representatives need to carry out corrective or preventive activities in relation to their medical devices. This corrective action can be initiated through the establishment post market information such as medical device’s complaints, incident reports and so on. It also includes the field corrective actions taken by the manufacturer to reduce the risk of harm to the patient, the operator or others and/or to reduce the re-occurrence of the incident.
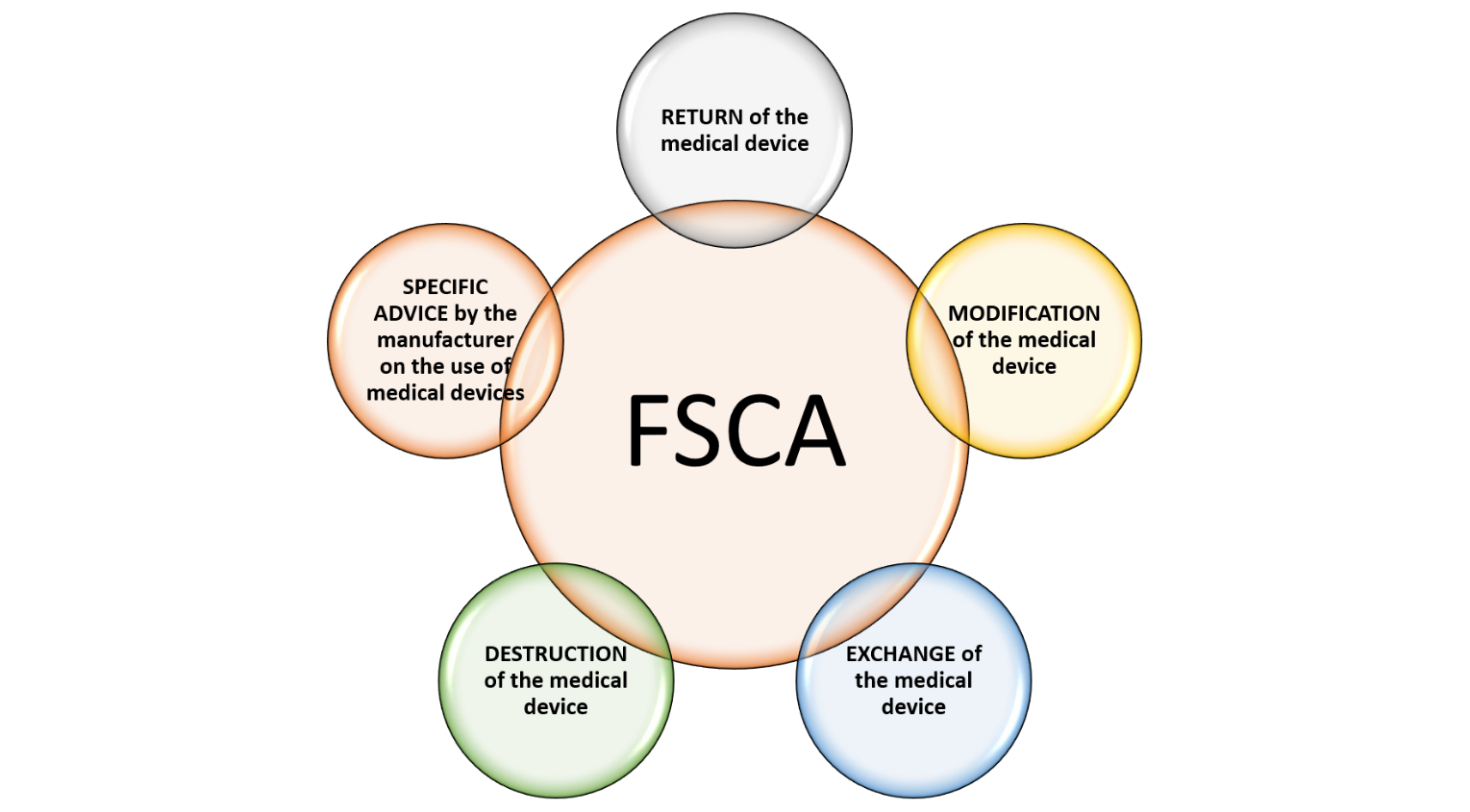
Field corrective action is an activity of action taken by the establishment to reduce the risk of incidents to enhance the safety and performance of a medical device. These actions may include:
 FIELD SAFETY NOTICE (FSN) is an important means of communicating a field safety corrective action (FSCA) and related safety information to users.
FIELD SAFETY NOTICE (FSN) is an important means of communicating a field safety corrective action (FSCA) and related safety information to users.
Process Flow of FSCA
For further information, kindly refer to the guidance document. MDA/GD/0013 Field Corrective Action.
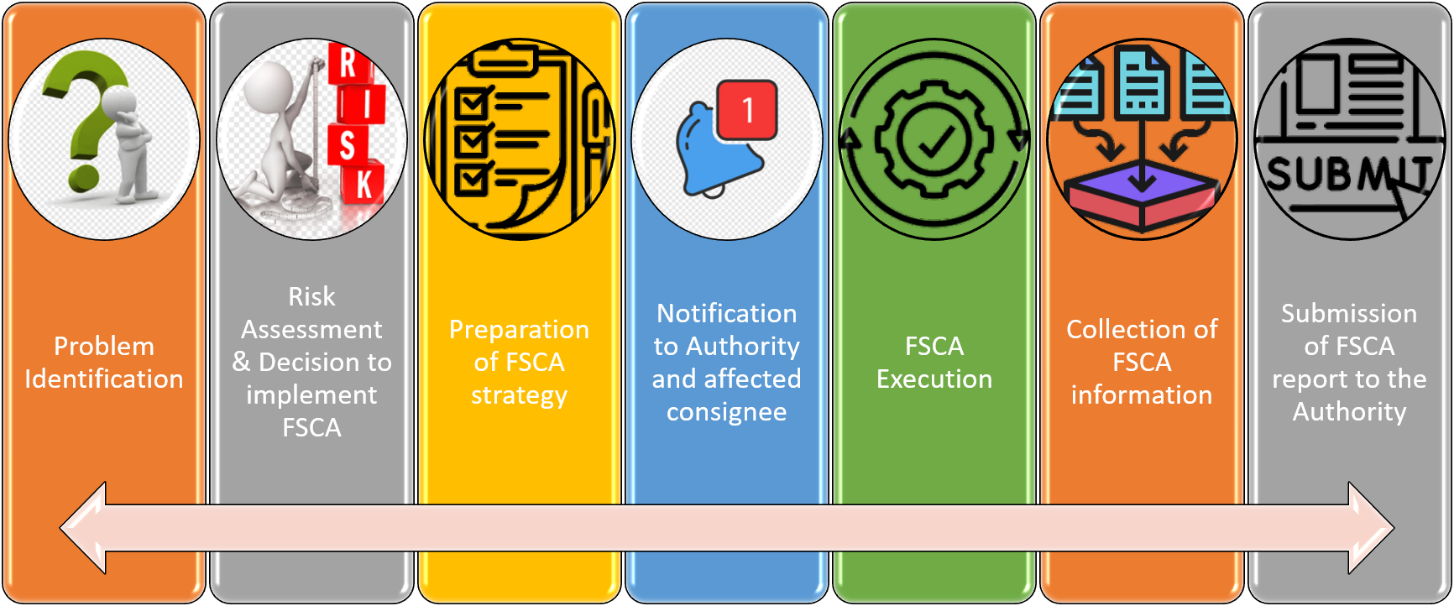
How To Notify FSCA?
Field safety corrective action (FSCA) may be requested by the Medical Device Authority for specific cases such as complaints and mandatory problem reporting (MPR). Field safety notice should be presented to the user and the Medical Device Authority before or during corrective action is taken by the establishments.
Field Safety Corrective Action (FSCA) for devices that affected Malaysia can be reported via an online system, Medical Device Centralized Reporting System, MeDCReSt
Contact Information
Updated: 20th February 2023






