ANNOUNCEMENT: IMPLEMENTATION OF MEDICAL DEVICE IMPORT PERMIT

MEDICAL DEVICE AUTHORITY
ANNOUNCEMENT: IMPLEMENTATION OF MEDICAL DEVICE IMPORT PERMIT
Dear Valued Clients,
The Medical Device Authority (MDA) serves as the designated Other Government Agency (OGA) responsible for issuing Import Permits (IP) for the importation of medical devices. The importation of medical devices is subject to import permit under the Customs (Prohibition of Import) Order 2023.
To support the implementation of this import permit, the MDA has taken the following measures:
✅Inclusion of medical device tariff codes in the Customs (Prohibition of Import) Order 2023;
✅Development of an ePermit System to facilitate import permit applications for medical devices and verification slip applications for non-medical devices that fall under medical device tariff codes; and
✅Preparation of guidelines and a user manual to assist importers in the application process for medical device import permit.
Procedure
Application for an import permit must be submitted through the online permit system (ePermit: http://newepermit.dagangnet.com.my). Applications may be submitted to the Authority at least 7 days prior to shipment of medical devices. Import permits will be processed within 3 working days from the application date.
For urgent shipments (e.g. overnight shipments by road from Singapore/Thailand or temperature-sensitive goods), the Authority may expedite processing to within 24 hours upon confirmation of payment.

Timeline
MDA has set the following key dates for implementation, as outlined in the timeline below:
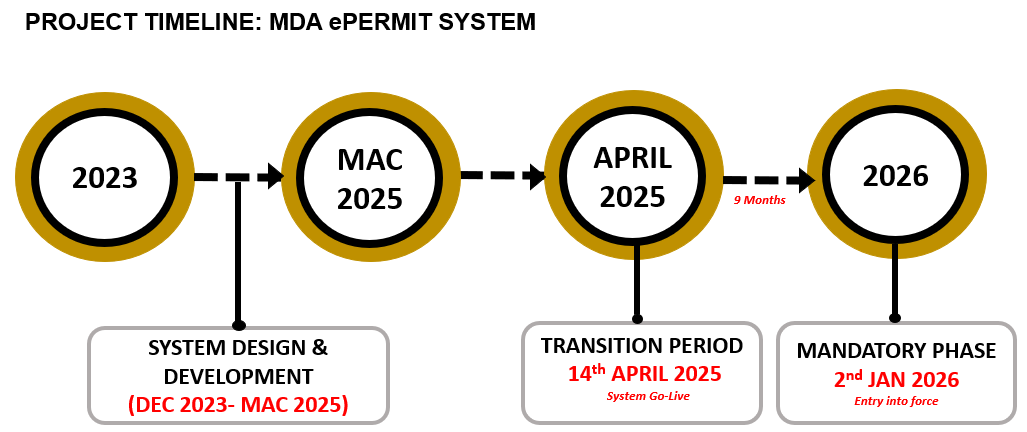
In order to ensure a smooth transition, all importers are encouraged to take the following actions during the transition period:
- Complete the registration of Super Users and Sub-Users.
- Familiarize themselves with the Import Permit application form to facilitate futuresubmissions.
Note: All importers are eligible for a 9-month processing fee waiver of up to RM130. However, the registration and annual subscription fees for the ePermit system still apply.
The Medical Device Authority (MDA) is gearing up to bring you industry awareness sessions and hands-on training. The schedules of the session are as follows:
|
Session |
Date/Time |
Mode |
Registration |
|
Import Permit Briefing Session Series 1 |
27 February 2025/ 10.00 am - 12.00 pm |
Physical |
Form(Limited to 50 pax)-CLOSE |
|
Import Permit Briefing Session Series 2 |
13 March 2025/ 10.00 am - 12.00 pm |
Virtual |
Form (Limited to 100 pax)-CLOSE |
|
Import Permit Briefing Session Series 3 |
16 April 2025/ 10.00 am - 12.00 pm |
Virtual |
Form (Limited to 100 pax) – CLOSE |
|
Import Permit Briefing Session Series 4 |
29 May 2025/ 10.00 am - 12.00 pm |
Virtual |
Form (Limited to 200 pax)- CLOSE |
|
Hands-on Training ePermit System- Series 1 |
16 Jun 2025/ 10.00 am - 4.00 pm |
Physical |
|
|
Hands-on Training ePermit System- Series 2 |
9 July 2025/ 10.00 am - 4.00 pm |
Physical |
|
|
Hands-on Training ePermit System- Series 3 |
7 August 2025/ 10.00 am - 4.00 pm |
Physical |
|
|
Hands-on Training ePermit System- Series 4 |
12 August 2025/ 10.00 am - 4.00 pm |
Physical |
Processing Fees for Permit
- The import permit fee is RM130 per application (applicable for all purposes except for sample investigation purposes)
- The verification slip fee is RM50 per application
Reference:
(1) Guideline: LINK
(2) User Manual: LINK
(3) List of HS Codes
The identified HS Codes that may be included in the gazettement: PDF Document
Contact details
- Mr. Luqman Hafifi :03-8230 0212
- Ms. Nur Aisyah: 03-8230 0253
- Contact number: 010-3323210 (Whatsapp only)
- E-mail: importpermit@mda.gov.my
- IT Call Centre: +603-8230 0222
- ITHelpdesk: https://ithelpdesk.mda.gov.my/
Contact details issues related to the ePermit system
- Careline DagangNet: 1300 133 133
- Email : careline@dagangnet.com
*CARELINE is available 24 hours daily, including public holidays
Please submit your survey below:
Thank you.
International Relations and Industry Division
Medical Device Authority (MDA)
Date of updated: 3rd June 2025






